no. of electrons in carbon|How many electrons in one mole of carbon dioxide? : Tuguegarao CO 2 is the carbon dioxide's chemical formula. The sum of the electrons in carbon and oxygen is used to compute the total number of electrons in carbon dioxide. One .
Tips for Playing Vegas Craps Games. By choosing any of the casinos above, you’ll already be off to a solid start with Vegas craps. However, you can improve your craps experience even more with the following tips. Be Properly Bankrolled for Large Odds Bets. Las Vegas offers up to 10x, 20x, and even 100x odds.
PH0 · What are the quantum numbers of carbon?
PH1 · The total number of electrons in one molecules of carbon dioxide
PH2 · The total number of electrons in one molecule of carbon dioxide is:
PH3 · The total number of electrons in one molecule of carbon dioxide is
PH4 · The total number of electrons in one molecule of carbon dioxide
PH5 · Number of Lone Pairs and Bonding Pairs for CO2 (Carbon dioxide)
PH6 · How many electrons in one mole of carbon dioxide?
PH7 · How many electrons in ONE MOLE of carbon dioxide?
PH8 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH9 · 2.2: Electron Configurations
PH10 · 1.8: Subatomic Particles
Tim Hortons NAIA Terminal 3 - 4th Level, Beside Foodhall Entrance, Ninoy Aquino (Manila) International Airport, Andrews Avenue, Pasay, Metro Manila, Philippines
no. of electrons in carbon*******In order to be neutral, an atom must have the same number of electrons and protons. Atoms—and the protons, neutrons, and electrons that compose them—are .
By Hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the .
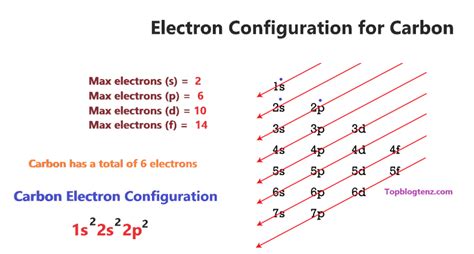
The element carbon (\(C\)) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms .
no. of electrons in carbon There is ONE CARBON ATOM, that is 6 electrons; and TWO OXYGEN ATOMS, that is 16 electrons, i.e. 22 electrons per molecule. And then we calculate the number of carbon dioxide .Step 1: Calculation of total electrons present in 1 molecule of Carbon dioxide. Carbon dioxide (CO 2), C has atomic numbers 6 so it has 6 electrons and oxygen has atomic .CO 2 is the carbon dioxide's chemical formula. The sum of the electrons in carbon and oxygen is used to compute the total number of electrons in carbon dioxide. One . To determine the number of lone pairs and bonding pairs of electrons for CO2 we first need to draw as valid Lewis Structure. Once we have a Lewis Structure f.The total number of electrons in one molecules of carbon dioxide is : 22. 44. 66. 88. A. 22. B. 88. C. 44. D. 66. Solution. Verified by Toppr. The correct option is A 22. Atomic .How many electrons in one mole of carbon dioxide? Carbon (atomic number #6#) can be found in the second row of the periodic table; at the ground state, the six electrons occupy two of its principle energy levels, .

3. Solution. Verified by Toppr. based on the electronic configaration of carbon, it has two unpaired electrons present. Hence, option B is correct. Was this answer helpful? 2. Similar Questions. Q 1. For C, Z=6. And if there are 6 nuclear protons, 6 fundamental, massive particles of unit positive charge within the nucleus, there must be 6 fundamental particles with unit negative charge, whizzing round the nucleus if the atom is neutral... And thus there are 6 electrons in a carbon atom...Carbon dioxide (CO 2), C has atomic numbers 6 so it has 6 electrons and oxygen has atomic number 8 means it has 8 electrons. Total electrons present= 16 + 6 = 22 electrons. Step 2: Calculation of the number of electrons in one mole of carbon dioxide. In one mole of atoms or electrons, there are 6. 022 × 10 23 number of atoms (Avogadro's .
Helium has 2 protons, 2 neutrons and 2 electrons. 3. Lithium has 3 protons, 4 neutrons and 3 electrons. 4. Beryllium has 4 protons, 5 neutrons and 4 electrons. 5.Calculate the number of electrons in 100 g r a m s of C O 2 Can a body have a charge of (a) 0.32 × 10 − 18 C (b) 0.64 × 10 − 20 C (c) 4.8 × 10 − 21 C ? Open in Appno. of electrons in carbon How many electrons in one mole of carbon dioxide? In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Carbon (C). From the Periodic .Carbon (C ), as a group 14 element, has four electrons in its outer shell. Carbon typically shares electrons to achieve a complete valence shell, forming bonds with multiple other atoms. Thus, the columns of the periodic table reflect the number of electrons found in each element’s valence shell, which in turn determines how the element . To determine the number of lone pairs and bonding pairs of electrons for CO2 we first need to draw as valid Lewis Structure. Once we have a Lewis Structure f.
Cheap Flight Deals from Denver to Tucson (DEN-TUS) Here are some of the best deals found on KAYAK recently from the most popular airlines for round-trip flights from Denver to Tucson that are departing in the next months. While these flights were available on KAYAK in the last 72 hours, prices and availability are subject to change and deals .
no. of electrons in carbon|How many electrons in one mole of carbon dioxide?